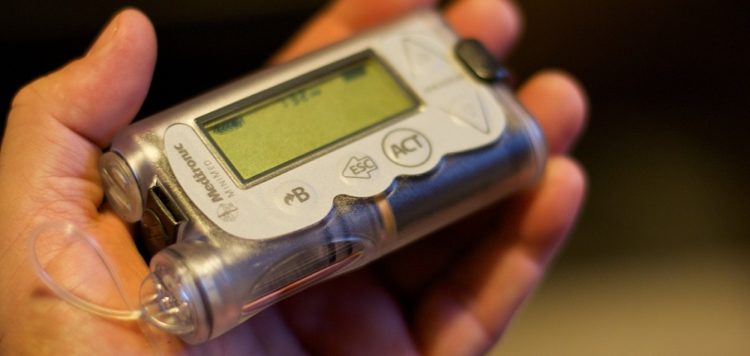
The recall is related to a series of vulnerabilities discovered by a team of cybersecurity researchers in 2018. In June 2019, the U.S. Food and Drug Administration (FDA) and Medtronic informed the public of a recall of MiniMed 508 and Paradigm series insulin pumps due to vulnerabilities that could allow an attacker to remotely hack the devices.
The FDA and Medtronic said that some affected users — whose devices were under warranty — were notified as early as August 2018.